Back to Jeff's Pages
The Yellow sodium "Proof"
Have you seen those
streetlights that put out yellow light?
 |
Here's a neat
demonstration but you need to do it with an adult. Take about a teaspoon
of table salt (sodium chloride, NaCl) and put it in a pile in the middle of a
heat-proof saucer.
Pour about a half of a teaspoon of rubbing alcohol over
the salt. Close the alcohol container and move it away. Now
CAREFULLY have the ADULT touch a lit match to the salt pile. Turn
down the room lights and enjoy the yellow glow! The yellow is from the
sodium ions in the salt.
See if you can "split" the light through a prism
or look at the flame's reflection using a CD.
You should see only 2 particularly brilliant yellow lines!
|
 |
You have done a
wonderful demonstration in chemistry and optics and explained some
history. In the past, tax collectors did this same test on home-made
whiskey in local taverns. By law, whiskey had to be at least 50% alcohol
and would be taxed accordingly. Whiskey with less than 50% alcohol will not
burn when poured on a salt pile. So if the whiskey on the salt did burn it
was "100% proved" legal; 50% alcohol. Even today, every bottle of drinking
alcohol, (gin, whiskey, vodka, etc.) is labeled in "proof"
which is twice the percentage of alcohol content. You "proved" rubbing
alcohol is at least 50% alcohol!

OK, so we made very bright
yellow flames from burning alcohol on a pile of
sodium Chloride
(salt).
And, if you looked at the light through a prism or reflected in a CD,
you saw the light was actually just 2 bright bars of yellow -- no red, green,
blue, or anything else.
"Bullets" of light and Electrons
Light (and x-rays, and
microwaves, and radiowaves) is made up of little packets, almost like bullets,
of energy called photons. Photons are made by electrons
and used by one electron to tell another electron about itself. So the
yellow light you saw in the sodium (salt/alcohol) flame was from an electron
saying,
"Hi! I'm an electron in the outermost
shell orbiting a sodium atom. A few seconds ago, somebody gave me a whole
bunch of energy
[that was you, your match, and burning alcohol]
and I
jumped out to a much bigger orbit! Well, I've cooled down somenow
and jumped down to my usual orbit. This yellow photon I'm sending you
tells you how far down I jumped."
Yup, that's how
electrons talk. Now, there was another electron in that sodium atom that
started in a slightly closer orbit, jumped up not quite as high, and gave off a
slightly lower energy yellow photon when it jumped back down to its
usual orbit. These were the 2 yellow lines you saw in the prism or
CD.
Every electron is
exactly like every other electron. The only thing that determines what
color light an electron gives off is how far "down" they jump (Quantum
Leap!).
Electrons can't just
jump any distance they want. The atom nucleus they orbit has rules about
just where it's electrons can orbit. So, a mercury atom usually
has 80 electrons in orbit. Each electron is in an orbit unique to
mercury.
When you see a green photon of exactly the right
shade of green (5460 Angstroms) you can say, "Hey! That photon came from an
electron around a mercury atom!"
Fingerprints for Gas?
Every kind of atom, from hydrogen (the lightest) to uranium
(the heaviest found naturally on Earth),
has an absolutely unique photon "signature". If you heat them
up, you get photons from their electrons. If you pass these photons
through a prism (or a "diffraction grating" like your CD) you get a bunch of
bright colored lines. Every kind of atom has a unique set of colored
lines. So, if you heat up a lump of anything and look
at the "spectrum" of colored lines given off, you can tell exactly what
the lump was made of! Nifty! This way of detected what something
is made of by looking at it's spectrum of colors is called
"spectroscopy".

If you take the light
from an incandescent lamp (not a fluorescent bulb!) like the kind Thomas Edison
invented and pass that light through a prism (or diffraction grating), you will
get your familiar continuous "rainbow" from red, orange, yellow, green,
blue, through violet. There will be no bright lines.

Here's the magic. If you take some cold sodium gas
(tricky to do but chemists can do this) and put those sodium atoms in
a clear glass jar, then
put the jar between the incandescent lamp and the prism, what will the spectrum
look like? You will see that same old "rainbow" of colors from red to
violet except there will be two black lines in
the yellow part of the rainbow and the 2 black lines
are in exactly the
same place as the 2 bright yellow lines when we burned our salt and
alcohol!!!!!
Cold atoms
absorb the exact same colors of light
that hot atoms emit!
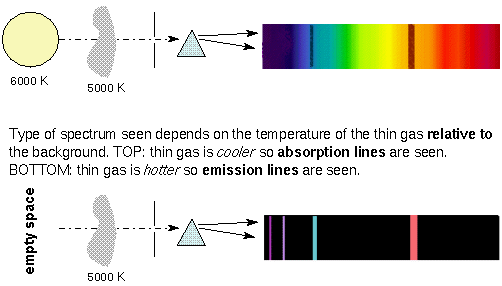
Absorption Lines
So far we've learned how every element has a unique set of bright,
colored spectral lines that are given off by heating up that element. Once
this element cools down it absorbs exactly the same colored spectral
lines.
These lines are called
Fraunhofer lines after Joseph Fraunhofer who, in 1817, was the second
scientist to observe them. If there was justice in the world, they would
be called Wollaton lines after the scientist who discovered them in
1802.
The spectrum
("rainbow") of the Sun has a whole bunch of dark absorption lines from
(relatively) "cool" gas in the Sun's outer atmosphere, the thin gas between the Sun and the
Earth, and the gas in our atmosphere.
Since we know what is
in our atmosphere, when we look at the dark spectral lines in the spectra from
distant stars, we can tell what kind of elements are in that star. We can
tell if the star is "old" or "young" or made from the gas from a previously
exploded star (just like you!).
But how can we use these
Fraunhofer absorption lines to figure out how far away a distant
galaxy is from us?
And how do we know about the "Big Bang",
and how we can tell if another star has planets orbiting it?
So far we have burned alcohol on salt to get yellow flames. We learned
that every element will turn to a gas if heated hot enough and
that glowing gas gives off very specific colors that show up as bright
"bars" if you pass the light through a prism. We also learn that if we
pass white light though "cold" gas and then pass the light through a prism,
you will see BLACK bars in exactly the same place in the "rainbow" where the
BRIGHT bars were when the gas was hot. These are called Fraunhofer
lines. Boy, we know a lot!
Ok, how does that help
us tell how big, how old, or how dense the Universe is?

